iData Global Courses
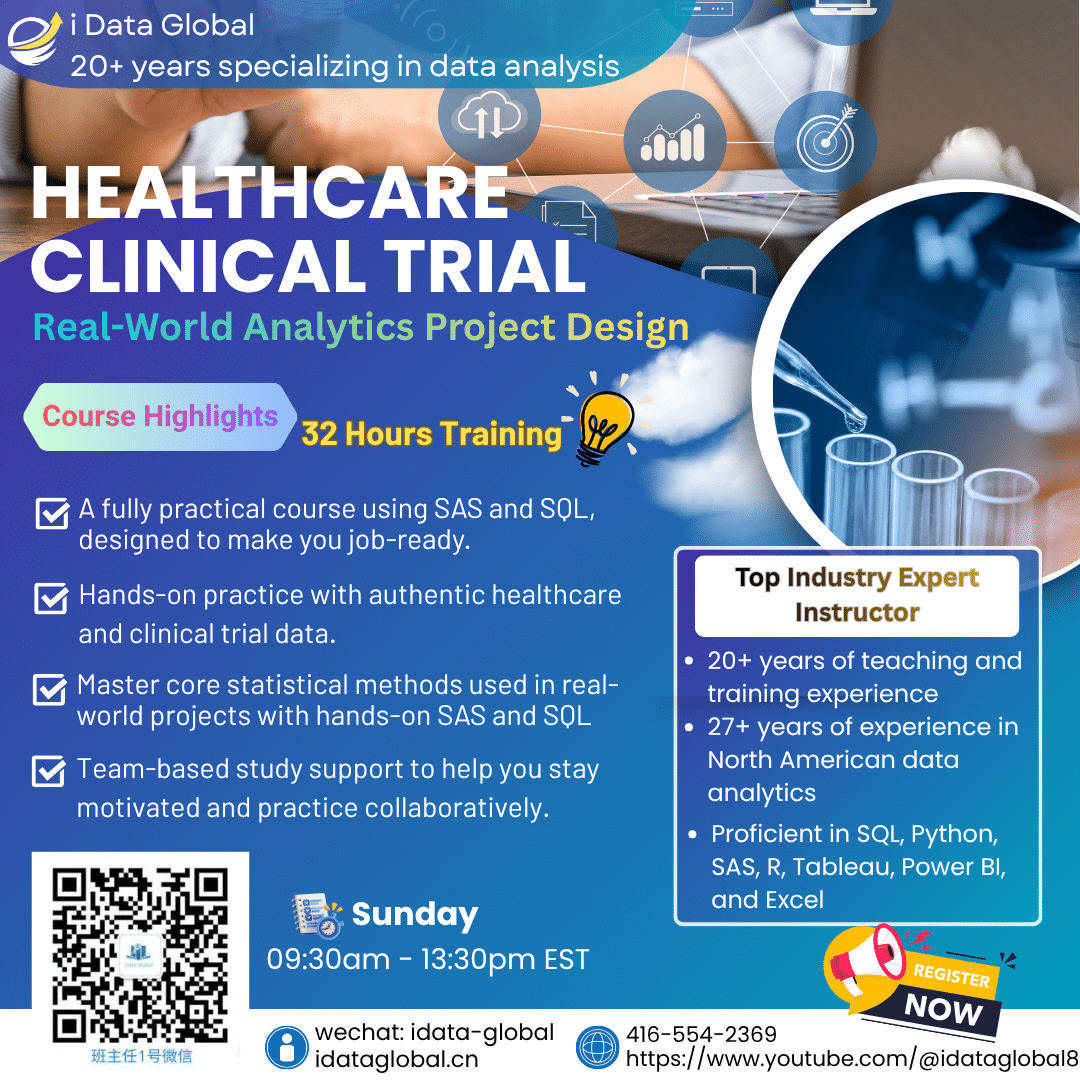
Clinical Data Analysis and Statistical Analysis (USING PHARMACEUTICAL DATA)
Clinical Data Analysis and Statistical Analysis (USING PHARMACEUTICAL DATA)
Teaching you statistics &reporting with pharma data. Reporting is an inevitable part of our job whenever we work with data, and it involves two requirements: what and how. Our pharma program teaches you comprehensive and detailed statistical analysis and reporting. We teach you comprehensive and detailed statistical analysis and reporting format/style. It also covers the handling of clinical trials and the standardization required by the FDA (SDTM, ADAM, etc.). The statistical analysis explained in this course can be directly applied to not only hospital/CRO/government clinical pharmaceutical data analysis, but also to marketing research, risk management.
Course Introduction
Clinical data analysis and statistical analysis (using pharmaceutical data)
Clinical Data and Statistical Analysis (Clinical Trial Focus)
Course Duration.
32 hours
Course Description:
This course is designed for participants who want to go deeper in the field of pharmaceutical data and clinical trial analysis. Using real pharmaceutical data, participants will learn in-depth statistical analysis, clinical data processing, and FDA-compliant report writing. The course covers basic statistical analysis to the management and analysis of clinical trial data, helping participants to master the skills of data analysis in hospitals, CROs (Contract Research Organizations), government agencies, and other scenarios, as well as in the areas of market research and risk management.
Course Features:
- Comprehensive Statistical Analysis Skills: In-depth explanations of basic and advanced techniques of statistical analysis help participants master how to process, analyze and interpret clinical trial data to support scientific and compliant business decisions.
- FDA-compliant report writing: Learn in detail to write FDA-compliant report formats (e.g., SDTM, ADAM) to ensure that data analysis results are presented clearly and accurately to meet clinical trial compliance needs.
- Clinical trial data management and analysis: Special attention is given to clinical trial data processing, including data cleansing, data formatting and data analysis, to ensure that data quality meets industry standards.
- Multi-disciplinary application: the skills learned can be widely applied in the fields of pharmaceuticals, market research, risk management, etc., expanding the career choices and application scenarios of the trainees.
Suitable for:
- Pharmaceutical data analysts: working professionals who want to improve their clinical trial data analysis and report writing skills.
- Zero-basic students: Beginners who are interested in clinical trial and pharmaceutical data analysis and want to enter the field.
- Career changers: those who are interested in switching to pharmaceutical data analysis or related fields.
- CRO Practitioners: Professionals working in contract research organizations (CROs) who need to master FDA standardization and clinical trial data processing skills.
Course Objectives:
Through the 32-hour course, participants will fully master the core skills of clinical trial data analysis, including statistical analysis, data processing and FDA standardized report writing. The course not only provides essential knowledge in the pharmaceutical industry, but also expands its application in areas such as market research and risk management, laying a solid foundation for success in clinical trial data analysis and other related positions.
Course Overview:
- Fundamentals and Advanced Techniques of Statistical Analysis: Introduces the basic concepts and methods of statistical analysis, and exercises using actual pharmaceutical and clinical trial data to help trainees master scientific analysis and interpretation methods.
- Clinical trial data processing and management: Detailed explanation of clinical trial data (clinical trial data) processing and management, including data cleaning, formatting and analysis, to ensure data compliance and accuracy.
- Report Writing and Presentation Skills: Participants will learn how to write professional statistical analysis reports, especially the FDA-compliant reports in SDTM and ADAM formats, to ensure data compliance in clinical trials.
- Multi-disciplinary applications and extensions: Participants will learn how to apply statistical analysis skills in pharmaceuticals, market research, risk management and other fields to meet the data analysis needs of different business environments.
Summary:
The “Clinical Data Analysis and Statistical Analysis (Using Pharmaceutical Data)” course provides participants with comprehensive statistical analysis and clinical trial data processing skills, especially the application of FDA standards. Through 32 hours of in-depth study, participants will be able to master the processing and analysis of clinical trial data, as well as write professional reports that comply with industry standards. These skills will significantly enhance participants’ competitiveness in the fields of pharmaceutical data analysis, market research, risk management, etc., and help them stand out in related positions.
Course Outline
Tool Introduction
Related Courses
-
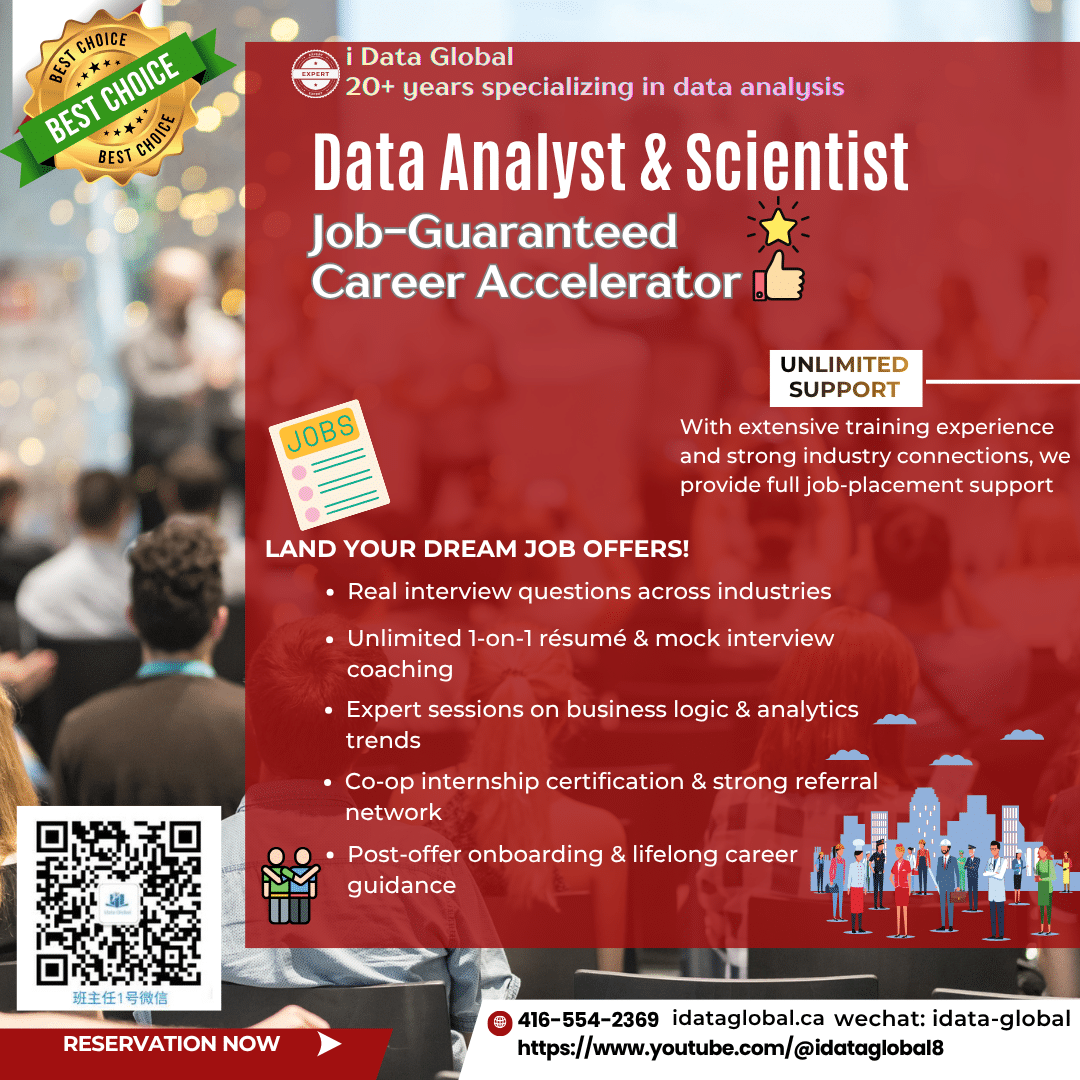
Data Analyst & Data Scientist Job-Guaranteed Career Accelerator
Flagship CourseQuick View -

AI | Machine Learning | Statistical Modeling | Big Data Analytics — The Ultimate Hands-On Bootcamp, Newly Upgraded!
Course Package 2 $1,999.00Quick View -
Data Analytics Project Design and Process (using financial data) /Project Design by Requirements
Course Package 2 $1,999.00Quick View